Kenya will begin rolling out lenacapavir, a new HIV-prevention drug administered twice yearly, across 15 priority regions in March, the health ministry announced on Wednesday.
Health Minister Aden Duale said the first phase of implementation would target 15 counties.
“The first phase of implementation will begin early March, covering 15 counties,” he said in a statement, adding, “We expect an additional 12,000 continuation doses by April.”
It has been demonstrated that lenacapavir lowers the risk of HIV transmission by over 99.9%. Given its frequent claims of being revolutionary, it is a chemical medication rather than a vaccine because it doesn’t elicit an immune response.
Kenya received its first batch of 21,000 doses on Tuesday through a deal involving Gilead Sciences, the drug’s manufacturer, and the Global Fund to Fight AIDS.
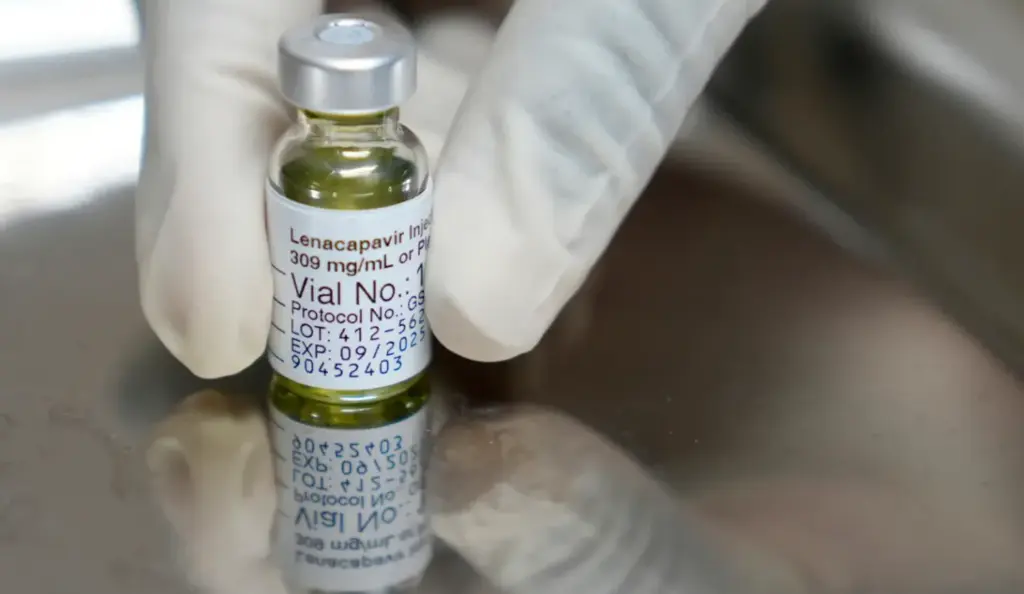
The United States government has also committed to supplying an additional 25,000 doses, according to the minister.
Kenya, where HIV prevalence stands at 3.7 percent, was among nine African countries selected last year to introduce lenacapavir.
Rollout has already begun in South Africa, Eswatini and Zambia since December.
Eastern and southern Africa account for approximately 52 percent of the 40.8 million people living with HIV worldwide, according to 2024 data from UNAIDS.
The introduction of lenacapavir comes as several African countries face reductions in aid from the administration of U.S. President Donald Trump, affecting HIV/AIDS programmes across the continent.
In December, Kenya signed a $2.5 billion health aid agreement with the United States, under which Washington will provide $1.6 billion over five years to support health initiatives, including combating HIV/AIDS and malaria and preventing polio.
Kenya is expected to contribute $850 million and gradually assume greater responsibility for programme funding.
The agreement, however, has been challenged in court by a Kenyan senator who alleges violations of the constitution.


 Trending
Trending