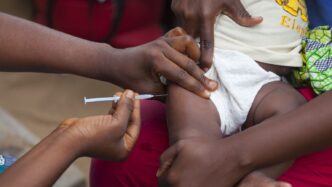
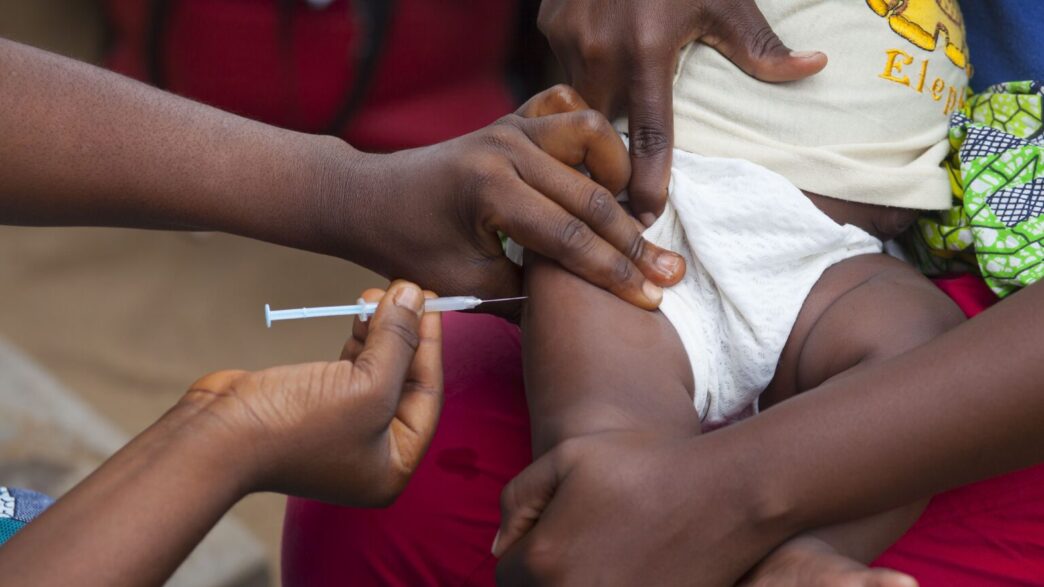
Guinea-Bissau has terminated a hepatitis B vaccine trial funded by the President Donald Trump administration, following condemnation from the World Health Organisation (WHO) and the international scientific community.
Foreign Minister Joao Bernardo Vieira said the study, which proposed giving the vaccine at birth to only half of participating newborns, was halted due to ethical concerns raised by scientists and US senators.
“It’s not going to happen, period,” Vieira told reporters on Tuesday.
The $1.6 million trial, approved by the US Centres for Disease Control and Prevention, was led by the Bandim Health Project at the University of Southern Denmark and aimed to enrol 14,000 infants to study potential “non-specific effects” of the vaccine, including skin and neuro-developmental disorders such as autism.
Under the study design, half the newborns would receive the hepatitis B vaccine at birth, while the remainder would get it at six weeks, the current standard. Some people argued that this exposed some infants to unnecessary risk.
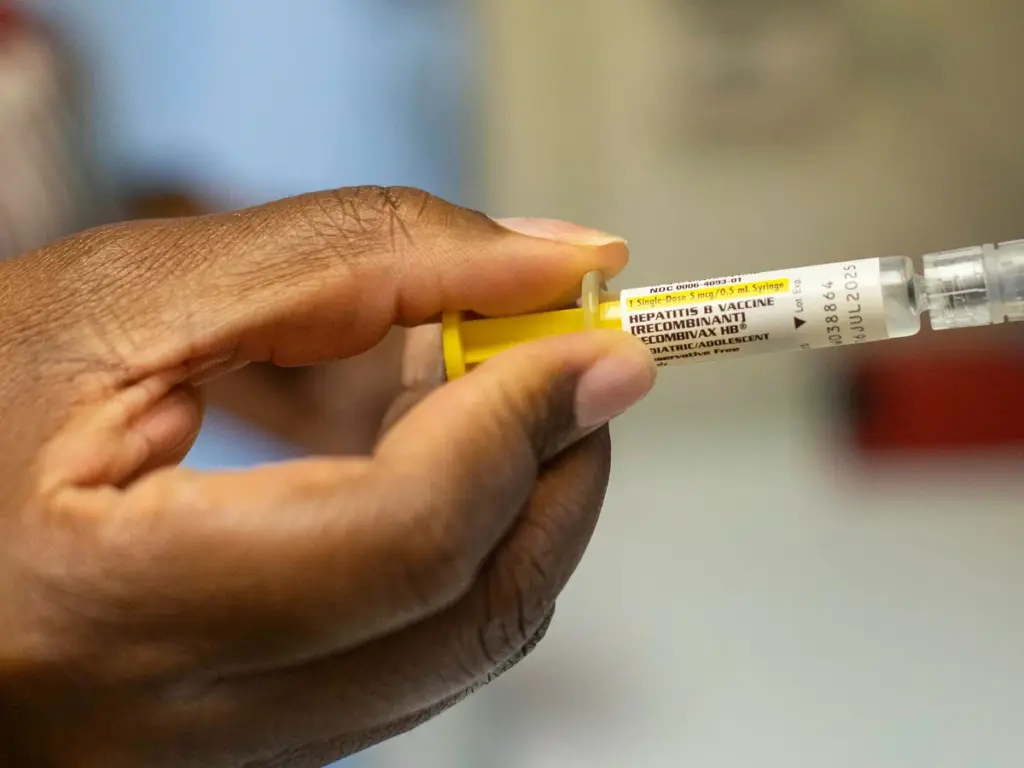
The WHO condemned the trial as unethical, saying that withholding a proven life-saving intervention from infants “exposes newborns to potentially irreversible harm.”
The organisation noted that the hepatitis B birth dose is an “effective and essential” public health measure with a well-established safety record.
Lead investigator Frederik Schaltz-Buchholzer defended the study, warning that halting it could undermine confidence in vaccines and health research.
“Everyone will lose if this trial is halted but, especially, confidence in vaccines and health research will suffer greatly,” Schaltz-Buchholzer said.
Schaltz-Buchholzer added that the group hopes to submit a revised trial proposal in the future.
The Bandim Health Project has operated in Guinea-Bissau for decades, studying the broader impact of vaccines to inform public health policy.


 Trending
Trending